X-chromosome inactivation is a fascinating biological process that poses unique challenges for female cells in balancing their genetic expression. Unlike males, who possess a single X chromosome, females have two, necessitating the silencing of one to prevent an overload of gene products. This intricate mechanism, pivotal in gene regulation, illuminates critical pathways for treating genetic disorders such as Fragile X Syndrome and Rett Syndrome. Pioneering research by Jeannie Lee at Harvard Medical School digs into the cellular dynamics of this phenomenon, which may ultimately enhance our understanding of chromosome studies and gene therapy advancements. These groundbreaking findings hint at innovative approaches to redesign therapeutic interventions, offering hope for individuals affected by X-linked mutations in the future.
The phenomenon of X-chromosome inactivation, often referred to as random X inactivation, plays a crucial role in gender-based genetic regulation. In females, where two X chromosomes exist, this process ensures a balance in gene dosage by silencing one of the X chromosomes—an essential mechanism for maintaining proper cellular function. The research led by Jeannie T. Lee has opened new avenues in the realms of Fragile X syndrome treatment and ongoing Rett syndrome research, shedding light on potential gene therapy advancements. By examining the molecular intricacies behind this chromosomal silencing, scientists are inching closer to breakthroughs that could revolutionize clinical approaches for genetic disorders. The intersection of chromosome studies and therapeutic innovation paints an exciting picture for future medical interventions.
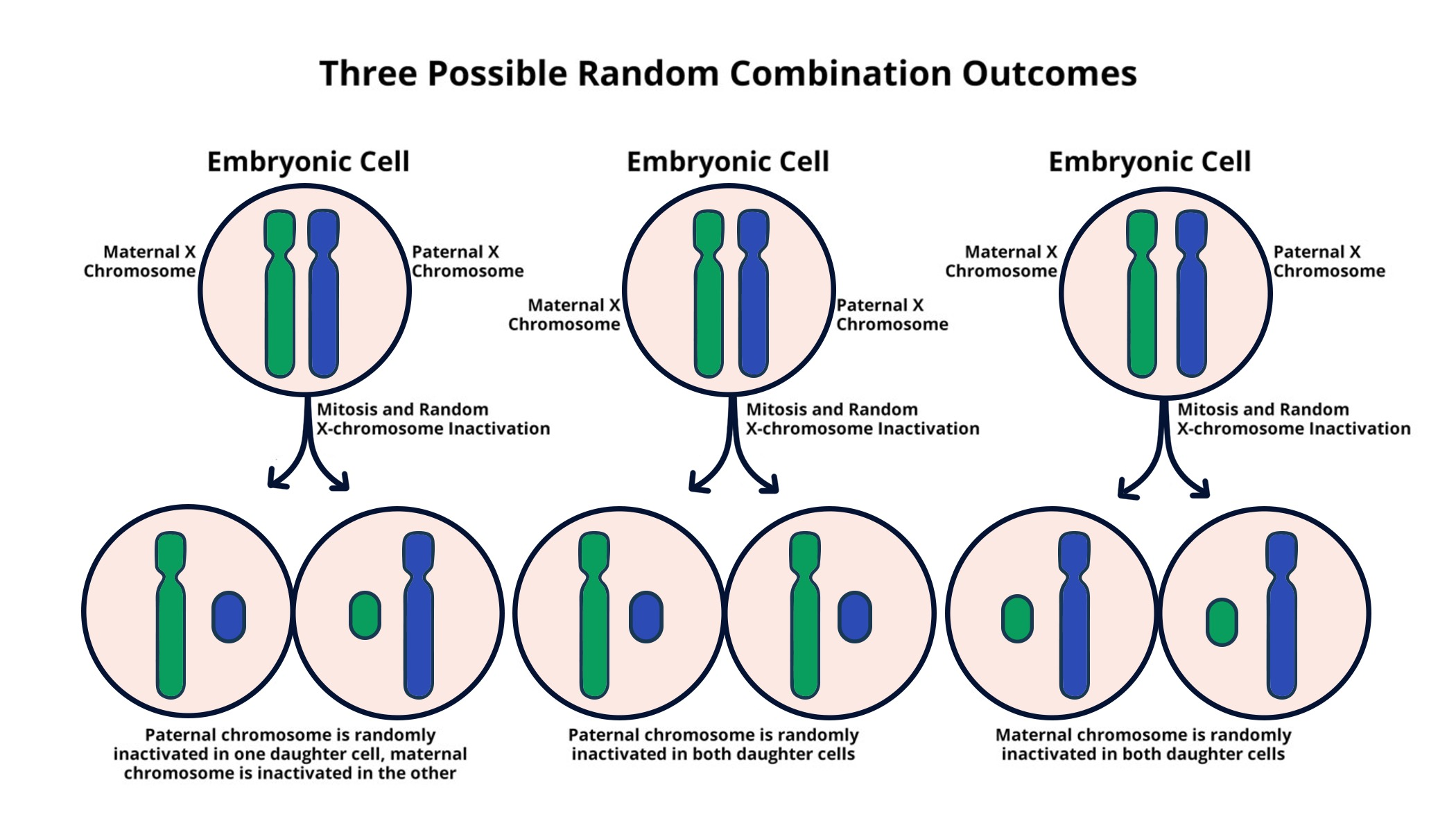
Understanding X-Chromosome Inactivation
X-chromosome inactivation (XCI) is an essential biological process that ensures dosage compensation between males and females due to the presence of two X chromosomes in females and only one in males. This intricate mechanism results in the silencing of one X chromosome in female cells, thus balancing gene expression levels. The research led by Jeannie T. Lee and her team at Harvard Medical School focuses on elucidating the complexities of this process. Their groundbreaking work sheds light on how certain chromosomal behaviors and the influence of RNA molecules, like Xist, play pivotal roles in achieving inactivation.
The implications of understanding XCI extend far beyond basic biology, as disruptions to this process can lead to various genetic disorders. Mutations on X chromosomes can result in conditions such as Fragile X Syndrome and Rett Syndrome, both of which are linked to intellectual and developmental disabilities. By comprehensively studying XCI, researchers hope to uncover potential therapeutic strategies to reactivate silenced X-linked genes. This work could pave the way for innovative treatments utilizing gene therapy advancements targeted towards mitigating the effects of these debilitating syndromes.
Breakthroughs in Fragile X Syndrome Treatment
Recent advancements in understanding the molecular mechanisms behind chromosomal inactivation have significant implications for developing treatments for Fragile X syndrome. Jeannie Lee’s research highlights a newfound approach to unsilencing genes that were previously thought irretrievable due to their inactivation on the X chromosome. The Jell-O-like substance or matrix surrounding chromosomes plays a critical role in how gene expression is regulated. With ongoing studies, researchers are testing compounds that could potentially enable the healthy version of the FMR1 gene responsible for Fragile X syndrome to become accessible in affected individuals.
Moreover, the promising results from Lee’s lab indicate that these treatment strategies could not only benefit females with Fragile X syndrome but may also extend to males affected by similar mutations. By reinstating the functionality of mutated X-linked genes, patients may see improvements in cognitive functioning and overall quality of life. As clinical trials loom on the horizon, the hope for effective interventions in Fragile X syndrome treatment moves closer to reality, thanks to foundational research paving the way for innovative gene therapy approaches.
Rett Syndrome Research: New Frontiers
Rett syndrome, predominantly impacting females, is characterized by normal early growth followed by a loss of acquired skills. Understanding the genetic underpinnings of Rett syndrome is crucial for developing effective treatments. Jeannie T. Lee’s continuous research on X-chromosome inactivation offers valuable insights into how mutations can lead to this neurodevelopmental disorder. By targeting the X-linked genes involved in Rett syndrome, researchers are investigating ways to activate these genes, potentially reversing symptoms of the disease.
The ongoing research at Harvard informs the scientific community about possible new avenues for drug development. Innovative therapies focusing on reactivating dormant X-linked genes hold promise for addressing Rett syndrome at its core. With funding from the National Institutes of Health, Lee’s lab is committed to exploring the biophysical alterations caused by agents like Xist in mitigating syndromic effects. The hope is that future treatments could restore function to genes that have been silenced, enhancing therapeutic options for individuals suffering from this complex condition.
The Role of Chromosome Studies in Disease Understanding
Understanding chromosome behavior in human cells is fundamental to grasping how many diseases manifest, particularly those linked to the X chromosome. Research studies conducted by experts, including Jeannie T. Lee, emphasize the importance of both classic and cutting-edge chromosome studies in uncovering the basis of genetic disorders. By investigating how genes are expressed or repressed on chromosomes, scientists can identify the root causes of diseases like Fragile X syndrome and Rett syndrome, which both result from mutations on the X chromosome.
As advances in chromosome studies evolve, the potential to translate these findings into therapeutic strategies increases exponentially. There is a burgeoning focus on how disruptions in X-chromosome inactivation processes can lead to various health complications. With ongoing research, gene therapy advancements are anticipated to emerge from these studies, offering hope to countless individuals impacted by chromosomal disorders and paving the path for innovative treatments that target specific genetic anomalies.
Gene Therapy Advancements in Genetic Disorders
Gene therapy has emerged as a revolutionary approach to treating genetic disorders by correcting faulty genes responsible for disease development. The recent advancements in this field have garnered attention, particularly regarding the treatment of X-linked conditions such as Fragile X syndrome and Rett syndrome. By utilizing the knowledge gained from X-inactivation studies, researchers like Jeannie T. Lee are exploring therapeutic avenues that may unsilence mutated genes by effectively manipulating the cellular environment, allowing for the healthy versions of these genes to express and restore function.
As gene therapy techniques continue to improve, their application in treating X-linked genetic disorders promises to change the landscape of medical treatment. Targeting the specific mechanisms that lead to gene silencing opens new pathways in medicine that could yield significant, long-term benefits for patients with untreatable conditions. With a focus on safety and efficacy, the journey towards implementing gene therapy for Fragile X syndrome and Rett syndrome is a critical milestone in the ongoing battle against genetic disorders.
Jeannie Lee: A Leader in Chromosomal Research
Jeannie T. Lee’s contributions to the field of genetics have positioned her as a leading figure in chromosome research, particularly in unraveling the complexities of X-chromosome inactivation. Her lab at Harvard Medical School has been at the forefront of this groundbreaking work, focusing on how chromosomal behaviors affect gene expression and contribute to various genetic disorders. As vice chair of the Department of Genetics, Lee’s research not only advances our understanding of fundamental genetic mechanisms but also emphasizes the potential for such knowledge to be translated into clinically applicable therapies.
Under Lee’s leadership, the lab has made significant strides in identifying the biophysical properties of the Jell-O-like substance surrounding chromosomes and its role in gene silencing. By systematically investigating these elements, her team is providing insights that could lead to targeted therapies for conditions like Fragile X syndrome and Rett syndrome. The continuous support from the National Institutes of Health has enabled Lee’s laboratory to pursue these critical questions, bridging the gap between basic science and therapeutic innovation.
The Clinical Potential of X-Chromosome Research
The clinical implications of Jeannie Lee’s research into X-chromosome inactivation are vast and promising. The ability to potentially unsilence inactivated X chromosomes holds the key to treating numerous genetic disorders linked to the X chromosome. This discovery could lead to innovative treatments that restore the function of essential genes rendered inactive due to chromosomal processes. As more studies substantiate the findings regarding how to safely reactivate silenced genes, the transition from laboratory research to clinical applications becomes increasingly realistic.
Emerging clinical trials utilizing the approaches developed in Lee’s lab may reshape the treatment landscape for individuals living with Fragile X syndrome and Rett syndrome. By translating basic genetic research into potential therapies, Lee’s work exemplifies how scientific advancements can lead to tangible health benefits. The ongoing investigations promise not only to enhance our understanding of X-linked disorders but also to provide hope and new treatment options for those affected.
Funding and Support in Genetic Research
Ongoing funding and support play crucial roles in advancing genetic research and facilitating breakthroughs in the understanding of complex genetic disorders. The National Institutes of Health has been instrumental in providing the necessary resources for Jeannie Lee’s lab to explore fundamental questions surrounding X-chromosome inactivation. This long-term commitment to basic research lays the groundwork for potential clinical applications that can dramatically improve patient outcomes for diseases linked to X chromosome mutations.
The collaboration between government-funded institutions and academic researchers ensures that innovative studies continue to emerge, driving forward the fight against genetic disorders. With increasing recognition of the importance of targeted research and development, the potential for funding initiatives to support promising avenues is integral to achieving scientific and medical milestones. Providing researchers with the tools and resources they need ensures that the advancements made today will yield positive impacts on genetic disorders like Fragile X syndrome and Rett syndrome tomorrow.
The Future of Genetic Research and Therapy
Looking ahead, the landscape of genetic research is ripe with potential as scientists strive to uncover the mechanisms underlying various genetic disorders. Jeannie T. Lee’s research on X-chromosome inactivation marks a significant step toward realizing the clinical applications of gene therapy, particularly for conditions like Fragile X syndrome and Rett syndrome. With ongoing studies exploring the potential to unsilence mutated genes, the future points toward a convergence of basic science and therapeutic innovation.
As more researchers join the effort to bridge gaps in understanding and treatment, the potential for transformative medical therapies increases. By continually pushing the boundaries of genetic research, scientists hope to develop safer, more effective approaches to gene therapy that address not just the symptoms but the root causes of genetic disorders. With collaborative efforts, sustained funding, and public support, the future of genetic therapy holds great promise in significantly enhancing quality of life for those affected by debilitating conditions.
Frequently Asked Questions
What role does X-chromosome inactivation play in Fragile X syndrome treatment?
X-chromosome inactivation is crucial in understanding Fragile X syndrome treatment because it helps researchers like Jeannie Lee explore ways to unsilence genes that are mutated. By focusing on how Xist alters the surrounding jelly-like substance, therapeutic strategies can target the inactivated X chromosome to restore gene function and potentially treat Fragile X syndrome.
How does understanding X-chromosome inactivation contribute to Rett syndrome research?
Understanding X-chromosome inactivation significantly contributes to Rett syndrome research by revealing mechanisms that can unsilence mutated genes. The work by Jeannie Lee’s lab explains how alterations in the X chromosome’s biophysical properties, through Xist, can help activate healthy versions of genes in patients with Rett syndrome, paving the way for gene therapy advancements.
What are the implications of X-chromosome studies for gene therapy advancements?
X-chromosome studies, particularly those focused on inactivation mechanisms, have vast implications for gene therapy advancements. By revealing how Xist modifies the chromosomal environment, researchers can develop targeted therapies that restore function to inactivated genes, offering hope for conditions linked to X-link mutations like Fragile X and Rett syndromes.
How does Jeannie Lee’s research on X-chromosome inactivation address chromosome studies?
Jeannie Lee’s pioneering research on X-chromosome inactivation is a key component of chromosome studies, aiming to unravel the complexities of gene expression regulation. Her findings on the gelatinous structure that facilitates X inactivation provide essential insights into how chromosomes interact and operate, which is vital for developing therapeutic strategies for X-linked disorders.
What potential breakthroughs have emerged from X-chromosome inactivation research at Harvard?
Potential breakthroughs from X-chromosome inactivation research at Harvard, particularly from Jeannie Lee’s work, include innovative approaches to treat diseases caused by gene mutations. By understanding the mechanisms that silence the X chromosome, these studies could lead to therapies that activate healthy genes, offering new treatment avenues for conditions like Fragile X syndrome and Rett syndrome.
| Key Topics | Details |
|---|---|
| X-Chromosome Challenge | Females have two X chromosomes, while males have one. Inactivation is necessary to balance gene dosage. |
| Role of Xist Gene | The Xist gene produces an RNA molecule that modifies the surrounding chromatin (‘Jell-O’) facilitating inactivation. |
| Mechanism of Inactivation | The interaction between Xist and the Jell-O leads to a flexible coating that silences the X chromosome. |
| Potential Drug Development | Research may lead to treatments for Fragile X Syndrome and Rett Syndrome by unsilencing inactivated X-linked genes. |
| Clinical Implications | Freeing the mutated genes while sparing many healthy genes suggests low side effects in potential therapies. |
| Long Research Journey | Decades of research culminated in a therapeutic breakthrough, demonstrating the relevance of basic research. |
Summary
X-chromosome inactivation is a crucial biological process that ensures females do not express double the amount of X-linked genes compared to males. Jeannie T. Lee’s research has shed light on the intricate mechanism behind this cellular phenomenon, offering potential therapeutic avenues to treat genetic disorders linked to the X chromosome. By understanding how the Xist gene interacts with the surrounding chromatin, scientists may soon develop targeted treatments for conditions like Fragile X and Rett syndromes, thereby revolutionizing the way we approach genetic disorders.