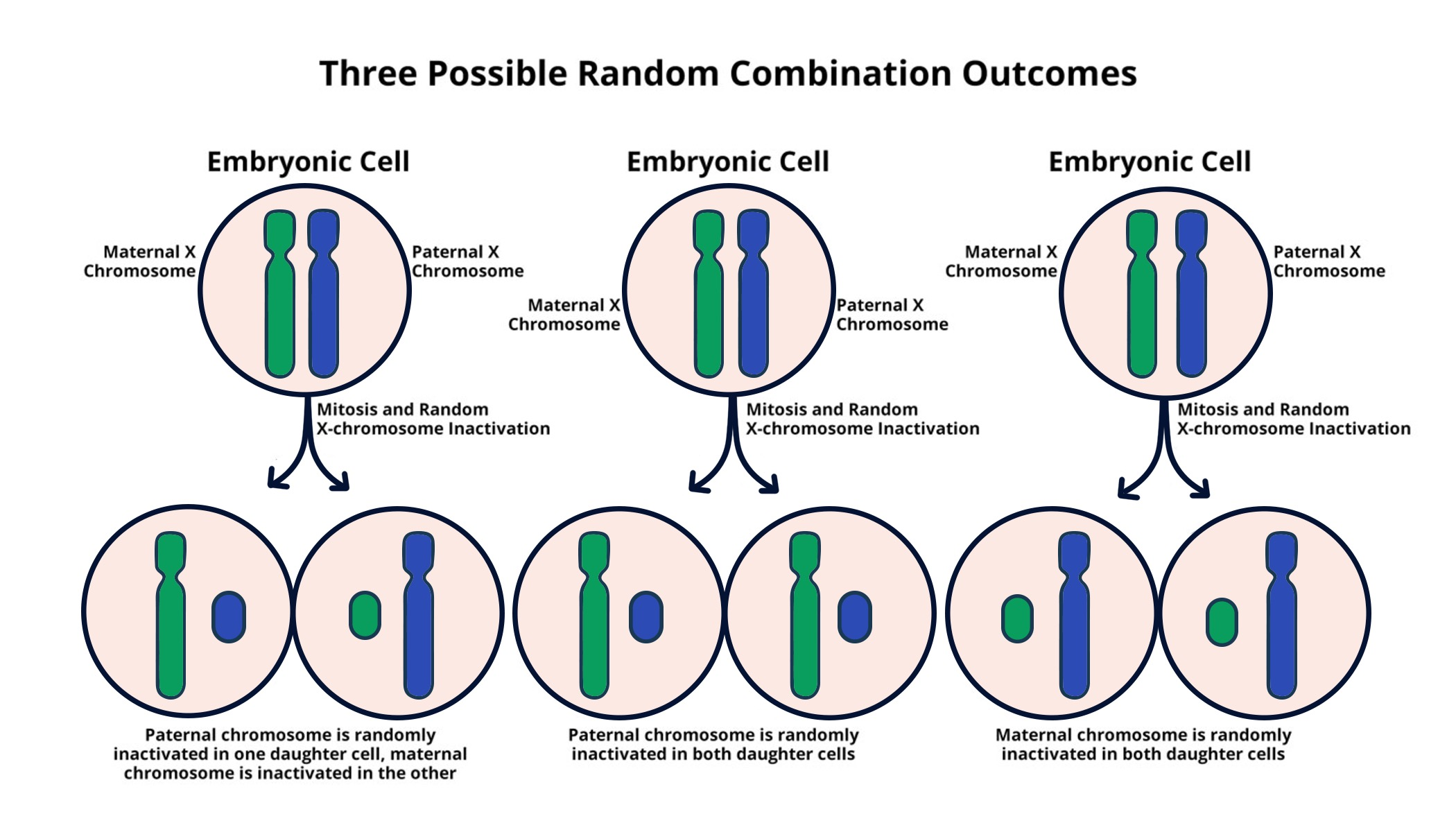
X chromosome inactivation (XCI) is a remarkable biological process that delicately balances gene expression in female mammals, where two X chromosomes reside, compared to males who have only one. This intricate mechanism ensures that cells do not operate with an excess of gene activity from the X chromosome, helping to prevent the phenotype imbalances seen in conditions such as Fragile X Syndrome and Rett Syndrome. By effectively silencing one of the X chromosomes, XCI plays a pivotal role in the progression of various X-linked genetic diseases. Recent advancements suggest that gene therapy targeting this process could pave the way for innovative treatments, offering hope to those affected by these chromosomal disorders. Understanding the dynamics of chromosomal silencing deepens our knowledge of genetic regulation and opens new avenues for therapeutic intervention in inherited conditions.
The phenomenon of X chromosome inactivation (XCI), also referred to as dosage compensation, has profound implications for genetic expression and health. In females, where two X chromosomes exist, this biological system is critical for ensuring stability in gene activity and preventing excess expression of X-linked genes. The study of this regulatory process has immense relevance for understanding disorders such as Fragile X and Rett syndromes, both of which stem from mutations on the X chromosome. Emerging therapies that influence the inactivation state of genes present new prospects for X-linked genetic diseases, potentially revolutionizing treatment options through innovative techniques such as gene editing and therapy. This growing body of research not only shines a light on chromosomal silencing mechanisms but also holds promise for targeted interventions that could alleviate the burdens of these complex genetic disorders.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a significant biological process that ensures dosage compensation of X-linked genes between males and females. In females, who have two X chromosomes, one is inactivated in each cell, a phenomenon that is crucial for proper cellular function. This arbitration of genes helps prevent disorders related to excess gene expression. The recent research conducted by Jeannie Lee and her team at Harvard Medical School has advanced the understanding of XCI, revealing the complex interactions between RNA molecules like Xist, and a gelatinous substance that encases chromosomes, aiding in chromosomal silencing.
The biological mechanisms of X chromosome inactivation involve a fascinating series of events where Xist RNA modifies the biophysical features of the surrounding chromosomal ‘Jell-O’. This process enhances the flexibility of the gel-like structure, enabling access to specific regions of the X chromosome for silencing. These insights are pivotal in understanding how diseases linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome, might be treated by unsilencing the inactive X, thereby allowing expression of healthy genes.
Frequently Asked Questions
What is X chromosome inactivation and why is it important?
X chromosome inactivation (XCI) is a biological process in which one of the two X chromosomes in female mammals is randomly silenced to equalize gene expression between males (who have one X chromosome) and females. This process is crucial for preventing an overdose of X-linked gene products in females and plays a significant role in conditions like Fragile X Syndrome and Rett Syndrome.
How does X chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is an X-linked genetic disorder caused by mutations in the FMR1 gene on the X chromosome. In females, if the normal FMR1 gene is on the inactivated X chromosome, it cannot express the healthy gene product, leading to the symptoms of the syndrome. Understanding X chromosome inactivation can help in developing gene therapy strategies to restore expression from the silenced X.
What are the implications of X chromosome inactivation for gene therapy?
Research on X chromosome inactivation has potential implications for gene therapy, particularly in treating X-linked genetic diseases like Fragile X Syndrome and Rett Syndrome. By developing methods to unsilence the inactivated X chromosome, it may be possible to reactivate the healthy genes present, offering hope for effective treatments.
What role does Xist RNA play in X chromosome inactivation?
Xist RNA is a crucial molecule that orchestrates X chromosome inactivation by coating the X chromosome and modifying its surrounding structure, or ‘chromosomal Jell-O.’ This process effectively silences the genes on the X chromosome, allowing researchers to explore ways to manipulate Xist for potential therapeutic applications.
Can X chromosome inactivation therapies benefit male patients with X-linked disorders?
Yes, therapies targeting X chromosome inactivation could potentially benefit male patients by restoring function to mutated genes on the X chromosome. While males do not undergo X inactivation, the mechanisms of gene silencing for specific mutations can be exploited to reduce symptoms associated with X-linked genetic disorders like Fragile X Syndrome.
What are some challenges in understanding X chromosome inactivation?
Despite advances, challenges remain in fully understanding the mechanisms of X chromosome inactivation. Researchers are still investigating how the inactivated X can be liberated to activate mutated genes without impacting the healthy genes present, as well as the reasons why some genes are unaffected during this process.
How has recent research advanced our understanding of X chromosome inactivation?
Recent research from Jeannie T. Lee’s lab has uncovered details about the mechanisms of X chromosome inactivation, particularly the role of ‘chromosomal Jell-O’ and Xist RNA. This work has opened new avenues for potential therapies for X-linked genetic diseases, benefiting those suffering from conditions such as Fragile X and Rett Syndromes.
What future directions do researchers see for X chromosome inactivation studies?
Researchers aim to further optimize methods of unsilencing inactivated X chromosomes and conduct safety studies. The ultimate goal is to move these potential therapies into clinical trials, which could lead to effective treatments for X-linked genetic diseases, improving the quality of life for affected individuals.
| Key Points | Explanation |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, but only one is typically active. The other is inactivated to balance gene expression between males and females. |
| Role of Xist RNA | The Xist RNA modifies the chromosomal environment, creating changes that lead to the inactivation of one X chromosome. |
| Potential Treatments | Research by Jeannie T. Lee’s lab may lead to therapies for genetic disorders like Fragile X and Rett syndromes. |
| Importance of ‘Jell-O’ concept | The gelatinous substance surrounding chromosomes, termed ‘Jell-O’, plays a crucial role in separating and inactivating the X chromosome. |
| Clinical Implications | Understanding X chromosome inactivation paves the way for potential therapies to un-silence inactivated X chromosomes, potentially helping those with genetic disorders. |
Summary
X chromosome inactivation is a vital biological process that ensures that females, who possess two X chromosomes, do not express double the amount of X-linked genes compared to males. This intricate mechanism involves the action of Xist RNA and the surrounding chromosomal environment, described as ‘Jell-O,’ which enables the inactivation of one of the two X chromosomes in females. Research by Jeannie T. Lee has opened new avenues for potential therapies targeting genetic disorders such as Fragile X and Rett syndromes, suggesting that by understanding and manipulating this process, it may be possible to restore function to mutated genes. The implications of this discovery are profound, highlighting the potential for significant advances in treatments for genetic diseases.